Diagnostics
Read more about Anti-Müllerian Hormone (AMH) Quality Control, RIQAS Anti-SARS-CoV-2 Serology EQA Programme, Molecular Controls for Infectious Disease etc.
Read more about Anti-Müllerian Hormone (AMH) Quality Control, RIQAS Anti-SARS-CoV-2 Serology EQA Programme, Molecular Controls for Infectious Disease etc.


Quality Control is our passion; we believe in producing high quality material that can help streamline procedures, whilst saving time and money for laboratories of all sizes and budgets. With an extensive product offering, comprising third party quality controls & calibrators, interlaboratory data management, external quality assessment, you can count on reliable results and peace in mind for your data quality managment.
New third-party Acusera controls:
Active Vitamine B12 Control for quantitative determination of Active Vitamin B12 in human serum and plasma.
Anti-Mullerian Hormone (AMH) Control for quantitative determination of AMH.
Brain Natriuretic Peptide (BNP) Control for unbiased, independent assessment of BNP in human serum and plasma.
Bone Markers (Serum) Control for determination of Procollagen Type 1 N-Terminal Propeptide (P1NP), N-MID Osteocalcin (OC), and Bone Alkaline Phosphatase (B-ALP) in serum samples.
Pre-Eclampsia Control for quantitative determination of placental growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) in human serum and plasma.
Serum Indices Control for detection of HIL interference (haemolysed, icteric or lipaemic samples) that can affect patient test results.
Ultra-low PSA Control for montoring performance of low levels of PSA.
Xanthochromia Control for monitoring Bilirubin and Oxyhaemoglobin in Cerebrospinal Fluid (CSF).


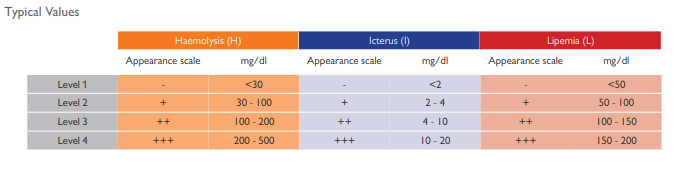
Identify pre-analytical errors in haemolytic, icteric and lipemic samples with the new Acusera Serum Indices Control.
The release of the new version of ISO 15189 (2022), introduces increased focus on risk stratification and mitigation for patients and laboratory stakeholders and more emphasis on quality control to improve accuracy and validity of results.
Interference caused by haemolysis, icterus and lipemia is responsible for many rejected results and incorrect diagnosis. It is therefore important to accurately capture the presence of interfering substances in samples before quantitative analysis is carried out.
HIL (Haemolysis, Icterus, Lipemia) interference can have detrimental effects on the determination of concentration of many analytes. It is therefore crucial to determine levels of HIL interference to improve laboratory efficiency and to reduce the frequency of erroneous results. HIL interference leads to poor test turnaround times and additional expenses as sample collection and analysis must be repeated in cases of haemolysis, icterus or lipemia in samples. More serious consequences
of poor HIL detection may be delayed or incorrect diagnosis of patient samples, leading to late or inappropriate treatment.

RIQAS is the world´s largest External Quality Assessment scheme with more than 55 000 laboratory participants spanning over 134 countries
RIQAS programmes provide early detection of test system errors, rapid user-friendly reports and flexible programme options.
Our new programmes this year:
Neonatal Bilirubin
Serologu (Anti-SARS-CoV-2)
Serum Indices
Cytokines
Microbiology (Bacterial Identification)
Anti-Müllerian Hormone (AMH)

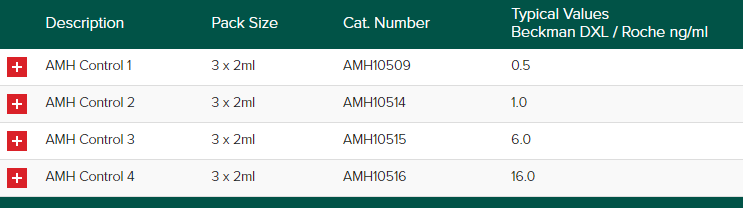
The Randox Acusera AMH control is designed to use as a third-party control for the quantitative determination of Anti-Müllerian Hormone (AMH). An AMH test is often used to check a woman’s ability to produce eggs that can be fertilized for pregnancy. The number declines as a woman gets older. AMH levels help show how many potential egg cells a woman has left. Thus, helping women to make informed decisions about their health.
Benefits

The new RIQAS Microbiology Programme includes the identification of micro-organisms to gram positive/negative, genus and speccies levels together with Antimicrobial Susceptibility Testing (AST) according to chosen guidelines.
RIQAS Microbiology Programme is available in a bi-monthly format with 3 samples distributed twice per year. Each strain is presented in a lyophilised pellet form within a self-contained tube containing a pre-filled ampoule for reconstitution, therefore helping to avoid cross-contamination.
Case studies are provided with each sample that have been manufactured with robustly characterised strains.

The Qnostics Molecular Controls will mimic real patient samples in molecular disgnostic processes. The controls are true kit-independent third-party, based on whole pathogen material.
Available in a range of formats, for many different disease states, Qnostics controls will validate your diagnostic protocols and ensure regulatory compliance in your laboratory.
Why using third party controls?
Qnostics controls are the perfect tool to:
Determine data accuracy and precision
Avoiding lot-to-lot variation
Minimizing data drift
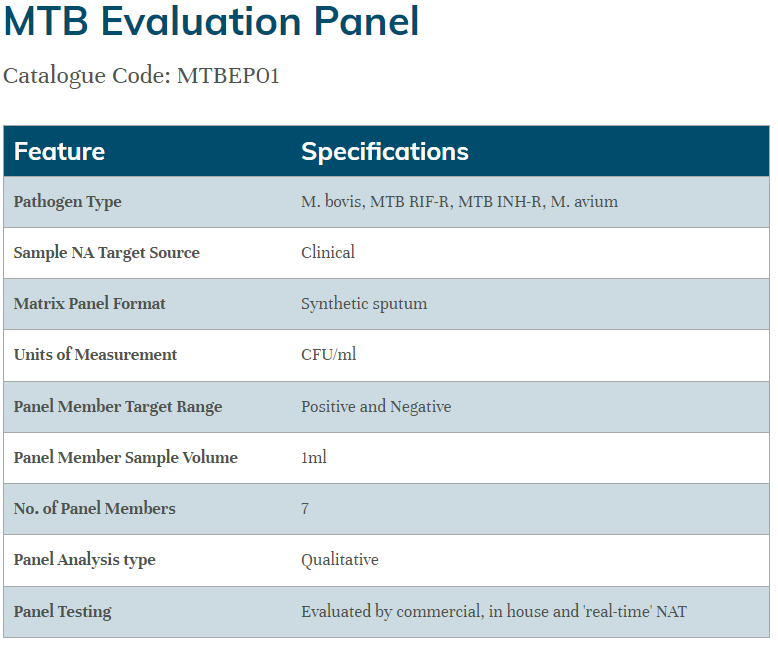
The Qnostics Mycobacterium tuberculosis controls offer a range of products for assessment of MTB molecular assays.
MTBQC01-B-RPBL3284-Rev01: third party control single-point positive controls for evaluation of assay repeatability
MTB Rifampicin-resistant Q Control: single point control for MTB/Rif-R
MTB Evaluation Panel: six data points plus negative covering M. bovis, M. avium, MTB/RIF-R (Rifampicin-resistant) and MTB/INH-R (Isoniazid-resistant)
MTB controls were tested to assess the performance of the panels on the Cepheid Xpert® MTB/RIF Ultra assay.

The SARS-CoV-2 Typing Evaluation Quality Control is suitable for use with most commercial and in-house methods for the evaluation of different variants of concern, molecular typing and nucleic acid sequencing.
The evaluation panels are manufactured to ISO standard 13485:2016. Each sample within the panel has been characterised using real-time PCR and Next Generation Sequencing (NGS).
Samples are representative of clinical human specimens in transport media (TM) with background human cells and are traceable to the first WHO International Standard for SARS-CoV-2 RNA (20/146) as well as an internal reference preparation in line with the requirements of ISO 17511:2020 using digital droplet PCR.


B. pertussis, B. Parapertussis, C. pneumoniae (TWAR), Influenza A
We are expanding the portfolio of multiplex quality controls for respiratory disease.
The Qnostics Respiratory Target Multiplex 6 Q Control is a panel contraining 5 positive controls. It is a combination of 4 common respiratory pathogens: Bordetella pertussis (BP), Bordetella parapertussis (BPP), Influenza A Type H3N2 (INFA H3) and Chlamydia pneumoniae (CP) in a single vial.
The panel consists of 5×0.7 ml, provided as liquid-frozen, ready-to-use. Target concentrations have been designed to be withing the dynamic range of most molecular assays and are consistent within each lot and across batches.


With more than 50,000 laboratory participants in over 139 countries, RIQAS is the world’s largest EQA scheme. We are pleased to announce the plan to expand our programme list with the introduction of a new full RIQAS anti-SARS CoV-2 Serology EQA programme. Comprising IgG, IgM and total antibodies, this new full scheme supports both qualitative and quantitative reporting.
The Qnostics Respiratory Target Multiplex (RTX) Controls consist of 5 independent control products covering a total of 20 different respiratory microbes. Each RTX Control is a combination of 4 different microbes in a physiological transport media for multiplex applications.
Qnostics offer whole pathogen based controls which will mimic the patient sample through the total extraction- and analysis process.
RTX controls are compatible with all commercial instrument platforms including rapid tests.
The RTX controls were designed for use with:
Cepheid Xpert® Xpress SARS-CoV-2/Flu/RSV assay o Only RTX1QC01 applicable
BIOFIRE® Respiratory Panel 2.1 plus
Qiagen QIAstat-Dx® Respiratory SARS-CoV-S Panel


We are pleased to announce a new QCMD EQA pilot study for SARS-CoV-2 (COVID-19) Antigen testing.
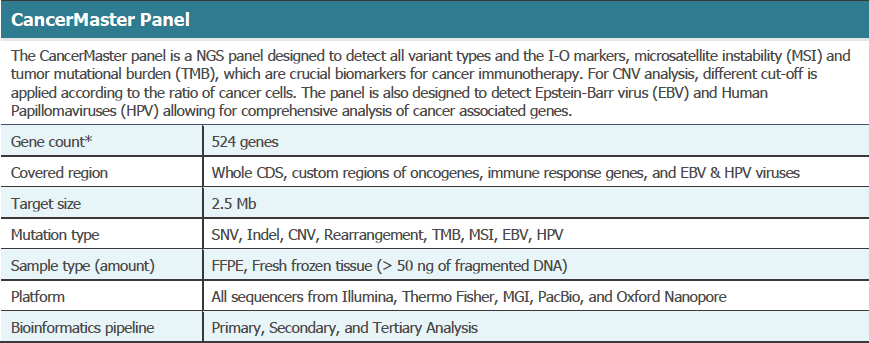
View the publication on predicting molecular features of chemotherapy and immunotherapy responses in advanced BTC´s using clinical sequencing.
The Cancer Master Panel provided:
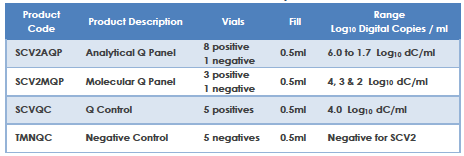

Qnostics’ range of SARS-CoV-2 (SCV2) products are designed to support laboratories in the verification, validation, and performance monitoring of their SARS-CoV2 molecular assay. The product range contains whole inactivated SCV2 virus covering the entire viral genome in a transport medium containing background human cells. The SCV2 materials have been extensively characterised across a wide range of different molecular workflows / assays both commercial and in house currently in use.
The SCV2 product range are external quality controls and are independent of manufacturers’ kit controls. These reagents enable laboratories using them to monitor assay, equipment and operator performance on a day to day basis.
The SCV2 product range fulfils the requirements of a reference material as described in ISO 15189.
Comprising both reactive and non-reactive controls for Anti SARS-CoV-2 , the Acusera SARS-CoV-2 Antibody Control will support assay validation and routine performance monitoring of serological assays for COVID-19. Conveniently supplied in a liquid ready-to-use format with a 30 day open vial stability, waste is kept to a minimum. As a true third party control, independent performance assessment is guaranteed.
Benefits

New Respiratory Target Multiplex (RTX) Controls
The Qnostics RTX control range enables the requirements for testing strategies during the upcoming flu season to be met. The range covers the viral and bacterial pathogens responsible for an array of respiratory diseases in various patient cohorts, whilst discriminating cold and flu from COVID-19.
RTX Plus Q Control
Target Pathogen – Influenza A (H1N1), Influenza B (Victoria), RSV A, Coronavirus (SARS-CoV-2)
RTX2 Q Control
Target Pathogen – Parainfluenza 1, Adenovirus 1, Mycoplasma pneumoniae, Coronavirus (OC43)
RTX3 Q Control
Target Pathogen – Parainfluenza 2, Metapneumovirus (A2), Enterovirus (A16), Coronavirus (229E)
RTX4 Q Control
Target Pathogen – Parainfluenza 3, Rhinovirus (16), Legionella pneumophila, Coronavirus (NL63)
RTX5 Q Control for paediatric testing.
Target Pathogen – Parainfluenza 4, Adenovirus (14), RSV B, Enterovirus (D68)

Randox Infectious Disease Serology Controls
User-friendly: Liquid ready-to-use and multi-analyte based, reducing the number of different control tubes in the lab
High quality: Human plasma-based with clinically relevant measuring values, manufactured under strict conditions
Economical: 60 day open vial stability for reduced waste
Analytes:
Lyme Disease: positive and negative, IgG and IgM
ToRCH: IgG positive, IgM positive, negative
Epstein Barr Virus, EBV: positive
HIV
Hepatitis
HTLV
Syphilis

Evidence Investigator Semi-automated Compact Benchtop System without Compromising on the Volume of Samples Processed
The Molecular Coronavirus Array simultaneously detects five strains of coronavirus including the COVID-19 (SARS-CoV-2, 229E, NL63, OC43 and HKU1). The wider panel provides a more comprehensive respiratory screen enabling informed treatment decisions to be made.
The new test utilises Randox Biochip Technology, with results available for 54 patient samples in less than 5 hours.
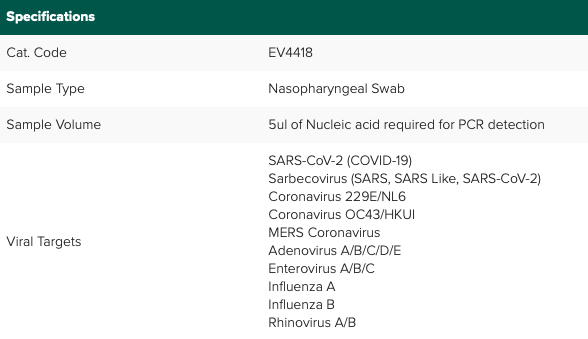
Sample Volume Investigator: 5μl of Nucleic Acid required for PCR detection.

New 4X 1Step RT-qPCR Kit for Detection of <5 Copies per Reaction
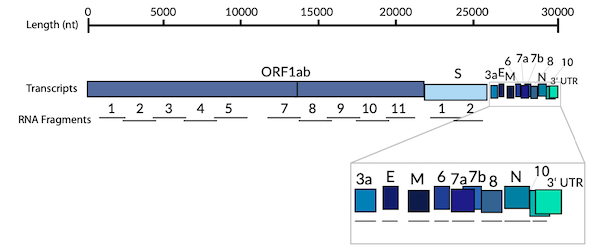
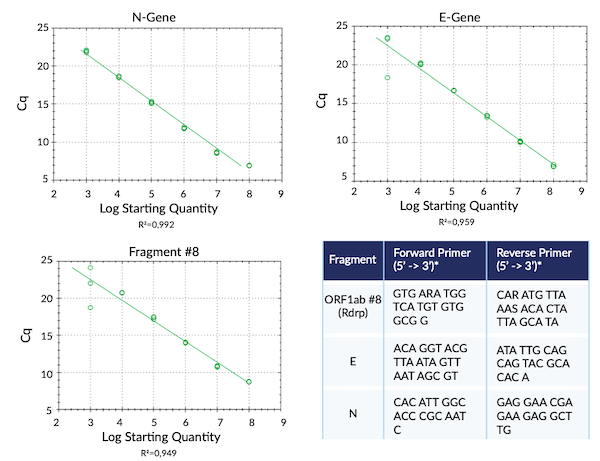
Complete SARS-CoV-2 Transcriptome in RNA Fragments


Qnostics controls from Randox Laboratories are independent 3rd party controls, specifically designed for molecular nucleic acid detection in infectious medicine testing.
Controls are based on whole pathogens, and designed to fully reflect the detection of patient samples.
You are able to assess the entire testing process from extraction though amplification and detection.
Easy-to-use; delivered in a liquid frozen format.
Manufactured to ISO 13485 quality standards.
Importance of 3rd party controls in molecular infectious disease testing
The following disease areas are covered:

Emergency Drugs of Abuse Screening with Multiplex Technology
Evidence Multistat is an automated benchtop immunoanalyser, using multiplex technology to enable more data from a single sample.

Why Use Control Reagents from an Independent Manufacturer?
Randox true third party controls are manufactured independently from reagents and calibrators. The unique value assignment process employs thousands of independent laboratories ensuring statistically valid targets are available for multiple instruments and methods.
With a shelf life of up to four years, laboratories can ensure continuity of lot supply whilst enabling long term QC monitoring. The availability of multi-analyte, multi-instrument controls will reduce costs and preparation time, consolidating multiple instrument specific controls into a single control product.
Want to get in touch?
We will support beyond today’s challenges, into future achievements!
Menu bar
Contact information
Follow us